CURIOX APPLICATIONS
TUMOR MICROENVIRONMENT
Standardize tumor sample preparation and reduce background for more accurate immunotherapy characterization.
- Home
- Applications _ Tumor microenvironment
Overview
Change the way you process cells to get reliably consistent data.
Interactions of cancer cells with their environment profoundly influences tumor progression, ultimately determining whether the primary tumor metastasizes, develops dormant micrometastases, or is eradicated. Thus, the tumor microenvironment (TME) profoundly shapes therapeutic responses and resistance. Tumor-infiltrating lymphocytes (TILs) possess the unique ability to recognize, attack and penetrate tumors. As such, mechanisms that unleash the power of TILs are under investigation as potential cancer treatments. For instance, mouse and human TILs are used in the preclinical studies of immunotherapeutic agents, while other human TILs are being developed and applied into novel immunooncology methods.
Hence, TIL isolation and further characterization are of key importance in assessing treatment efficacy in various tumor types. However, the downstream processing from TME encounters a common problem. Typically, after tissue dissociation and isolation, TILs share their samples with high amounts of debris and dead cells in solution, contaminants needing stringent washing for removal. Instead of subjecting these precious TILs to the high mechanical stress of centrifugation that further impact retention, Laminar Wash technology offers a gentle alternative that can improve yield and data quality, particularly of rare cell types in samples containing low initial cell counts.
“Due to its underlying principle, the Laminar Wash (system) means less mechanical stress on cells and less cell loss from a sample, compared to conventional centrifugation steps.”
Dr. Christoph Eberle, Charles River Laboratories
Traditional centrifugation
VS
Laminar flow technology
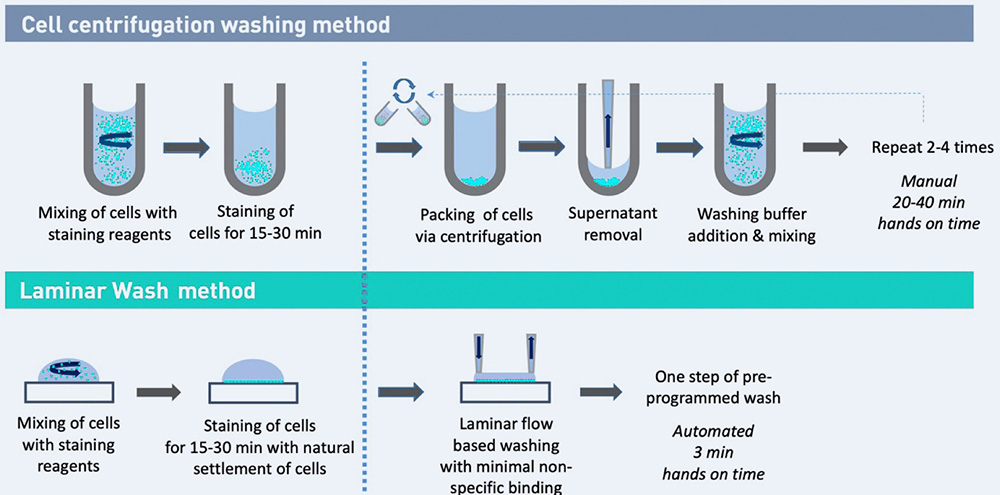
Comparison of an immuno-staining workflow for the preparation of a single cell suspension for flow cytometry analysis. Laminar Wash technology is equally effective in washing but without the use of a centrifuge.
Benefits
Benefits of laminar wash for tumor microenvironment samples
Tumor microenvironment and TILs isolation are difficult to prepare. Laminar Wash processing yields a clean sample while reducing hands-on time. Laminar Wash technology can:
- Enhance cell retention
- Improve reproducibility between operators, time, and locations
- Improve cell viability and reduce stress on cells
- Reduce debris, background, artifacts and clogging
- Offer facile solutions to automate cell prep workflows
Featured Resource
WHITE PAPER: Improved sample preparation method for immuno-oncology studies
Scientific Data
Laminar wash technology improves debris removal, tils retention, and resolution of subpopulations
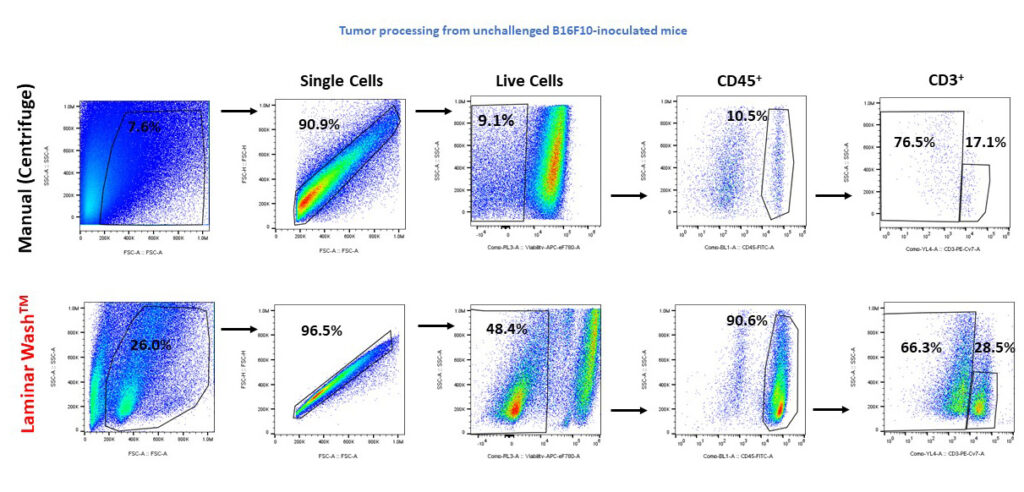
Data adapted from the Curiox Biosystems webinar by Dr. Christoph S. Eberle from the Discovery Research Services at Charles River Laboratories, Worcester, MA Better debris removal and cell retention:
When performing cell dissociation in tumor bearing tissues, the tissue debris and enzymatic-induced fragments are primary factors affecting TILs cell recovery. For more invasive and immunologically challenging models, like B16F10-inoculated mice, recovery of limited numbers of TILs reduces the chances for accurate characterization. Here, an unchallenged mouse sample containing 1e6 TILs was prepared with Laminar Wash technology. Laminar Wash improved viable cell counts and reduced debris, compared to conventional methods. The cleaner sample preparation increases lymphocyte recovery up to 90.6%, compared to centrifuge-harsh washing of 10.5%.
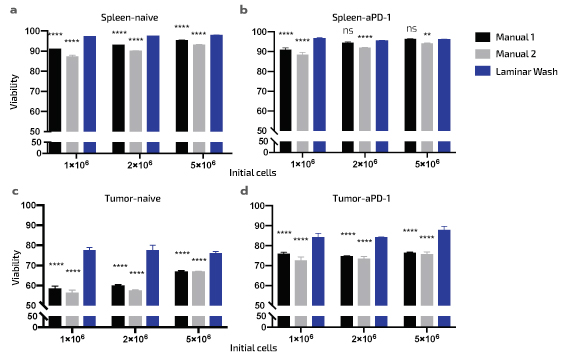
Laminar Wash Technology Improves Cell Viability
Samples processed with Laminar Wash show higher viability
Splenocytes (a) and (b) and dissociated tumor cells (c) and (d) were washed with either centrifugation (manual 1 and 2) or the Laminar Wash system and viability measurements were compared. Statistical significance is reported among the manual methods vs Laminar Wash: ns = not significant, ** = P<0.01, *** = P<0.001, **** = P<0.0001. The values represent technical triplicates of the samples from an
individual naïve and an individual challenged Balb/c mouse. Manually processed samples were handled by two different analysts..
Figure adapted from Curiox Biosystems and Charles River Laboratories co-authored White Paper
RELATED PRODUCTS AND ACCESSORIES
Curiox Laminar Wash
LAMINAR WASH™ HT2000 SYSTEM
Achieve higher throughputs and cleaner data with our 96-well laminar washing system.

Curiox Laminar Wash Auto
LAMINAR WASH™ AUTO 1000 SYSTEM
Accelerate high-throughput flow and mass cytometry workflows with fully automated sample preparation.


